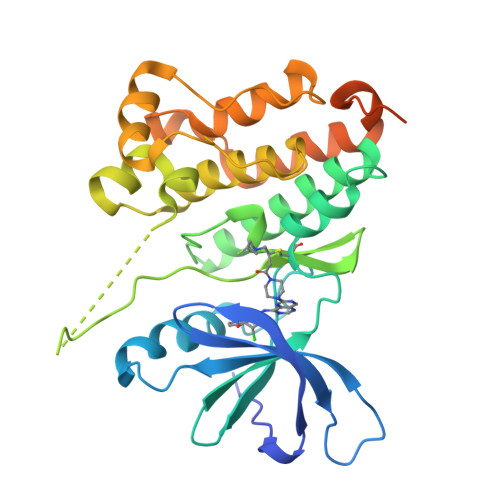
Discovery of potent and selective HER2 inhibitors with efficacy against HER2 exon 20 insertion-driven tumors, which preserve wild-type EGFR signaling.
Wilding, B., Scharn, D., Bose, D., Baum, A., Santoro, V., Chetta, P., Schnitzer, R., Botesteanu, D.A., Reiser, C., Kornigg, S., Knesl, P., Hormann, A., Koferle, A., Corcokovic, M., Lieb, S., Scholz, G., Bruchhaus, J., Spina, M., Balla, J., Peric-Simov, B., Zimmer, J., Mitzner, S., Fett, T.N., Beran, A., Lamarre, L., Gerstberger, T., Gerlach, D., Bauer, M., Bergner, A., Schlattl, A., Bader, G., Treu, M., Engelhardt, H., Zahn, S., Fuchs, J.E., Zuber, J., Ettmayer, P., Pearson, M., Petronczki, M., Kraut, N., McConnell, D.B., Solca, F., Neumuller, R.A.(2022) Nat Cancer 3: 821-836
- PubMed: 35883003
- DOI: https://doi.org/10.1038/s43018-022-00412-y
- Primary Citation of Related Structures:
7PCD - PubMed Abstract:
Oncogenic alterations in human epidermal growth factor receptor 2 (HER2) occur in approximately 2% of patients with non-small cell lung cancer and predominantly affect the tyrosine kinase domain and cluster in exon 20 of the ERBB2 gene. Most clinical-grade tyrosine kinase inhibitors are limited by either insufficient selectivity against wild-type (WT) epidermal growth factor receptor (EGFR), which is a major cause of dose-limiting toxicity or by potency against HER2 exon 20 mutant variants. Here we report the discovery of covalent tyrosine kinase inhibitors that potently inhibit HER2 exon 20 mutants while sparing WT EGFR, which reduce tumor cell survival and proliferation in vitro and result in regressions in preclinical xenograft models of HER2 exon 20 mutant non-small cell lung cancer, concomitant with inhibition of downstream HER2 signaling. Our results suggest that HER2 exon 20 insertion-driven tumors can be effectively treated by a potent and highly selective HER2 inhibitor while sparing WT EGFR, paving the way for clinical translation.
Organizational Affiliation:
Boehringer Ingelheim RCV, Vienna, Austria. birgit.wilding@boehringer-ingelheim.com.